Glutathione (GSH), as the most abundant non-protein thiol compound in cells, plays an irreplaceable role in the basic processes of maintaining life activities.
This tripeptide molecule composed of glutamic acid, cysteine and glycine, with its unique thiol group (-SH) as the core functional group, constitutes the first line of defense of the cell's antioxidant defense system.
GSHWORLD will systematically analyze the molecular characteristics and biosynthetic pathways of Glutathione, deeply explore its multi-dimensional physiological functions such as REDOX balance, detoxification metabolism, and immune regulation, and comprehensively review the current application progress of glutathione in disease treatment, agricultural stress resistance, nanomedicine and other fields. And focus on analyzing the innovative treatment strategies based on the regulation of glutathione metabolism and their transformation achievements.
basic concept and molecular characteristics of glutathione
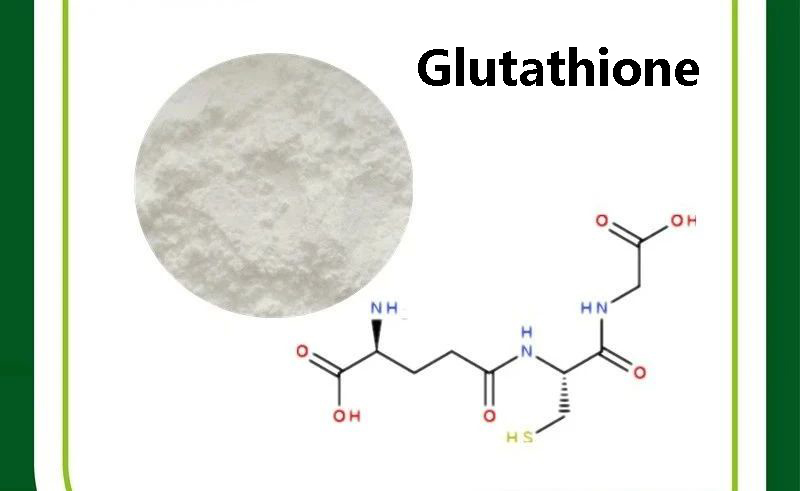
Glutathione (GSH) is a low-molecular-weight thiol tripeptide that is widely present in eukaryotic and prokaryotic cells. It is composed of glutamic acid (Glu), cysteine (Cys), and glycine (Gly) linked by peptide bonds, with the molecular formula C10H17N3O6S and a molecular weight of approximately 307.3 daltons.
This seemingly simple molecular structure actually contains complex biological functions. Its core active site lies in the thiol group (-SH) on the cysteine residue. This group endows glutathione with a powerful electron-donating ability, making it one of the most important reducing agents and antioxidant substances within cells.
The concentration of glutathione in mammalian cells is usually maintained within the range of 1 to 10 mM, and this high concentration reflects its fundamental position in the physiological activities of cells.
From the perspective of chemical properties, glutathione has the following notable characteristics:
The high reactivity of sulfhydryl groups enables them to rapidly combine with free radicals, heavy metal ions and various electrophilic substances.
The atypical peptide bond formed by the γ -carboxyl group of glutamic acid and cysteine enables it to resist degradation by most proteolytic enzymes.
The characteristics of amphoteric ions endow them with good water solubility, facilitating their free diffusion between the cytoplasm and organelles.
Inside cells, glutathione mainly exists in two forms: the reduced form (GSH) and the oxidized form (GSSG). The dynamic balance between the two (GSH/GSSG ratio) is regarded as an important indicator reflecting the REDOX state of cells. Under normal physiological conditions, this ratio is usually maintained above 100:1.
The biosynthesis of glutathione follows an ATP-dependent two-step enzymatic reaction pathway:
The first step is catalyzed by γ -glutamylcysteine synthase (GCL, including the catalytic subunit GCLC and the regulatory subunit GCLM) to form γ -glutamylcysteine (γ-GC) from glutamic acid and cysteine.
The second step involves the condensation of γ-GC with glycine catalyzed by glutathione synthase (GS) to form GSH.
This synthetic process mainly occurs in the cytoplasm, but there are also independent synthetic systems within the mitochondria.
It is worth noting that the availability of cysteine is usually the rate-limiting factor for GSH synthesis, which is why N-acetylcysteine (NAC), as a cysteine precursor, is often used to increase intracellular GSH levels.
The metabolic regulatory network of glutathione is very complex, involving multiple key transcription factors and signaling pathways.
Nuclear factor E2-related factor 2 (Nrf2) is the most important regulatory hub, which upregulates the expression of various GSH metabolism-related genes such as GCLC, GCLM and glutathione transporter by binding to antioxidant response elements (ARE).
Under oxidative stress conditions, Nrf2 dissociates from the Keap1 complex and translocates to the nucleus, initiating the transcription of a series of antioxidant genes and forming a positive feedback regulatory loop.
Transcription factors such as p53, NF-κB and AP-1 are also involved in the fine regulation of GSH levels.
From the perspective of subcellular distribution, glutathione is not only present in the cytoplasm but also localized to organelles such as mitochondria, endoplasmic reticulum, and nucleus through specific transporters (such as members of the SLC25 family), forming a compartmentalized distribution.
Especially in mitochondria, although it accounts for only 10-15% of the total GSH in cells, it is crucial for maintaining the integrity of mitochondrial function.
Studies have shown that under disease conditions such as diabetic retinopathy, the down-regulation of mitochondrial GSH transporter (Slc25a10/a11/a39) expression can lead to a decrease in mitochondrial GSH levels, thereby causing mitochondrial dysfunction and apoptosis.
The degradation of glutathione is mainly accomplished through the "γ -glutamyl cycle "mediated by γ -glutamyl transpeptidase (GGT), a process that not only participates in the turnover of GSH but is also closely related to the recovery of cysteine and the transmission of REDOX signals.
The high expression of GGT on the cell membrane surface enables the effective utilization of extracellular GSH, a characteristic that is particularly prominent in the tumor microenvironment. Some tumor cells obtain GSH secreted by adjacent cells by upregulating GGT activity to meet their high antioxidant requirements.
multi-dimensional functional mechanism of glutathione
Glutathione exhibits astonishing functional diversity in organisms, and its mechanism of action runs through all levels of cellular metabolism, forming a precise and complex antioxidant defense network.
As the most abundant non-protein thiol compound in cells, the primary function of GSH is to maintain REDOX homeostasis.
In this role, GSH participates in the clearance of peroxides and lipid peroxidation products by directly neutralizing reactive oxygen species (ROS) such as superoxide anion (O₂⁻), hydrogen peroxide (H₂O₂), and hydroxyl radicals (·OH), or by acting as cofactors of glutathione peroxidase (GPX) and glutathione reductase (GR).
It is worth noting that members of the GPX family (especially GPX4) play a core role in the regulation of ferroptosis. They utilize GSH to reduce lipid peroxides to the corresponding alcohols, preventing iron-dependent lipid peroxidation chain reactions.
When GSH is exhausted, the activity of GPX4 is lost, leading to ferroptosis. This mechanism has been used to design anti-cancer strategies that promote tumor cell death.
detoxification function constitutes second core role of glutathione
In the liver and other tissues, GSH is catalyzed by glutathione S-transferase (GST) to bind with various electrophilic substances (including environmental toxins, drug metabolites and endogenous harmful substances), forming more water-soluble GSH conjugates, which are easier to be excreted through bile or urine.
This process is particularly important for the detoxification of heavy metals such as cadmium, mercury, and lead. The thiol group of glutathione can directly chelate these metal ions, reducing their toxicity.
A research team from Hainan University discovered that in patchouli under cadmium stress, exogenous addition of glutathione could significantly improve the stomatal closure caused by cadmium, enhance the activity of antioxidant enzymes, and form a triple protective mechanism (regulation of photosynthetic pigments, activation of the antioxidant system, and regulation of metabolic pathways), effectively alleviating the toxic effects of cadmium on plants.
In the mammalian system, glutathione has also been proven to protect the kidneys from oxidative damage caused by nephrotoxic drugs such as gentamicin.
REDOX modification of protein sulfhydryl groups:
Glutathione is involved in a post-translational modification process of proteins known as "protein S-glutathionylation", that is, under oxidative stress conditions, GSH covalently binds to the active cysteine residues of proteins through mixed disulfide bonds.
A research team from Shandong University has recently revealed a new mechanism of this phenomenon in Escherichia coli:
In the presence of glutathione, five different oxidants (S₈, Cu²⁺, H₂O₂, ClO⁻ and NO donor) mainly induce glutathione modification (P-SSG) of the transcriptional regulator MarR of the multidrug resistance protein family, rather than tetramerization as traditionally believed.
This modification significantly weakens the binding affinity of MarR to DNA, promotes the expression of downstream genes, and represents a novel regulatory mechanism for bacteria to respond to oxidative stress.
In eukaryotic cells, protein S-glutathiylation is also involved in the regulation of multiple signaling pathways, including NF-κB, HIF-1α and Nrf2, etc., affecting cell proliferation, apoptosis and inflammatory responses.
In terms of immune regulation
Glutathione participates in the regulation of immune responses by influencing lymphocyte activation and cytokine secretion.
Studies have shown that a decrease in GSH levels is closely related to impaired T cell function, chronic inflammatory states, and the occurrence and development of autoimmune diseases.
The latest research by a team from the First Affiliated Hospital of Fujian Medical University has found that glutathione peroxidase 1 (GPX1) plays a key role in two common comorbidities in the elderly, namely frailty and hypertension.
As a GSH-dependent antioxidant enzyme, the activity changes of GPX1, by influencing REDOX balance, fatty acid and amino acid transport metabolic pathways, serve as a common molecular mechanism connecting the two diseases.
This study also screened out chickpeas A and epigallocatechin gallate (green tea extract) as potential GPX1-targeted regulators through molecular docking, providing new ideas for the intervention of related diseases.
Glutathione also plays a dual role in regulation of cell proliferation and apoptosis
On the one hand, a sufficient level of GSH is a necessary condition for cell cycle progression, especially during the DNA synthesis phase (S phase), where GSH provides a reducing equivalent for nucleotide reductase and supports DNA replication.
On the other hand, GSH depletion can trigger multiple programmed cell death pathways, including apoptosis, necroptosis and ferroptosis.
A collaborative study by Dalian Institute of Chemical Physics and the National Cancer Institute of the United States has revealed that in glioma cells carrying isocitrate dehydrogenase 1 (IDH1) mutations, the glutathione anabiotic pathway is abnormally active and regulated by the Nrf2 transcription factor.
After inhibiting Nrf2 activity with Triptolide, the expression of GSH synthesis-related genes such as GCLC, GCLM and SLC7A11 was down-regulated, and intracellular oxidative damage increased, ultimately leading to tumor cell apoptosis.
This discovery provides a new targeted strategy for the treatment of IDH1-mutated glioma.
It holds a unique position in energy metabolism and cell survival
Mitochondrial glutathione (mtGSH) serves as a special subcellular pool.
Research from Wayne State University School of Medicine shows that in a high-glucose environment, the expression of mitochondrial glutathione transporters (Slc25a10/a11/a39) in human retinal endothelial cells (HRECs) decreases, leading to a decline in mtGSH levels, mitochondrial structural damage and functional disorders, and ultimately causing cell death.
The reduction of mtGSH in the retina and the down-regulation of transporter expression were also observed in the diabetic mouse model, which provides a new explanation for the pathogenesis of diabetic retinopathy and suggests that targeting mtGSH transport may be a potential strategy for protecting the retinal health of diabetic patients.
application progress of glutathione in Disease treatment
The application of glutathione in clinical medicine has expanded from traditional detoxification and liver protection to multiple fields such as tumor treatment, neurological diseases, kidney protection, and anti-aging, demonstrating broad therapeutic prospects.
With the deepening understanding of the glutathione metabolic pathway and the advancement of drug delivery technology, precision treatment strategies based on glutathione regulation are becoming a hot topic in translational medicine research.
From simple glutathione supplementation therapy to complex metabolic interventions and nanodelivery systems, this field has achieved a series of remarkable clinical translational results.
Targeted therapy for glioma represents a significant breakthrough in glutathione metabolism intervention in field of oncology
A research team led by Xu Guowang from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, in collaboration with the National Cancer Institute of the United States, has found that glioma cells carrying isocitrate dehydrogenase 1 (IDH1) mutations are highly dependent on the glutathione metabolic pathway to maintain their survival.
This mutation leads to the production of a large amount of 2-hydroxyglutaric acid (2-HG) within tumor cells, triggering a unique metabolic remodeling that makes the anabolism of glutathione abnormally active under the regulation of Nrf2 transcription factors.
The research team used Triptolide, a natural compound extracted from Tripterygium wilfordii, an efficient Nrf2 inhibitor. Both in vitro and in vivo models confirmed that it could significantly inhibit Nrf2 transcriptional activity and down-regulate the expression of glutathione synthesis-related genes such as GCLC, GCLM and SLC7A11. Disrupt the REDOX balance of tumor cells and eventually induce apoptosis of cancer cells.
This innovative strategy provides a new approach for the treatment of IDH1-mutated glioma, and the related results have been published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
It is worth noting that, unlike traditional chemotherapy, this method targeting glutathione metabolism has selective toxicity and has a relatively small impact on normal cells because IDH1-mutated tumor cells are abnormally sensitive to oxidative stress.
In field of kidney diseases
The renal protective effect of glutathione has been supported by multiple clinical studies.
A clinical observation conducted by Xianyang Central Hospital in Shaanxi Province on 120 patients with hemorrhagic fever with renal syndrome (HFRS) complicated with acute renal failure (ARF) showed that the treatment group treated with reduced glutathione (1.8g/day intravenous infusion) on the basis of conventional comprehensive treatment had a cure and improvement rate of 95%, significantly higher than the control group's 86.7%.
The recovery time of renal function in the treatment group was significantly shortened (an average of 3.2 days vs. 3.8 days in the control group), and the number and frequency of cases requiring hemodialysis were also significantly reduced.
Studies have confirmed that glutathione can effectively eliminate excessive free radicals in patients with HFRS, protect renal tubules from damage by oxygen free radicals, reduce kidney damage, and increase the success rate of rescue.
More cutting-edge is that a research team from Assiut University in Egypt innovatively combined glutathione with the medicinal plant Lepidium sativum through nanotechnology to develop a new type of nanomaterial for gentamicin-induced acute renal failure.
This study utilized chitosan nanoparticles (CS NPs) loaded with GSH and combined them with spanlastics nanoparticles, demonstrating a synergistic renal protective effect in animal models.
Nanoformulations not only significantly improve renal function indicators (such as serum creatinine and urea nitrogen), but also simultaneously regulate oxidative stress (reduce MDA and increase SOD), inhibit the expression of inflammatory factors (TNF-α, IL-6), and reduce renal cell apoptosis (decreased expression of caspase-3) through multi-target actions.
This nano-co-delivery system overcomes the shortcomings of low oral bioavailability of GSH and poor stability of plant active components, providing a new paradigm for the treatment of drug-induced kidney injury.
Liver diseases are a classic application field of glutathione
Clinical studies as early as 2004 showed that the use of glutathione (trade name Atomoran) before and after resection of primary liver cancer could significantly shorten the recovery time of liver function (an average of 3.5 days in the treatment group vs. 7 days in the control group).
The mechanism lies in that GSH can neutralize the free radicals produced by surgical stress, alleviate ischemia-reperfusion injury, and promote hepatocyte regeneration and functional recovery.
In diseases such as non-alcoholic fatty liver disease (NAFLD), drug-induced liver injury and viral hepatitis, glutathione also shows a good liver-protecting effect, which is closely related to its multiple functions of promoting toxin excretion, inhibiting lipid peroxidation and regulating inflammatory responses.
prevention and treatment of diabetic complications is an emerging field of glutathione application
The research of the Wayne State University School of Medicine in the United States focuses on the dynamic regulatory mechanism of mitochondrial glutathione (mtGSH) in diabetic retinopathy.
Research has found that in a high-glucose environment, the expression of mtGSH transporters (Slc25a10/a11/a39) in human retinal endothelial cells decreases, leading to a decline in mtGSH levels, mitochondrial structural damage and functional disorders.
Overexpression of Slc25a10/a11 can improve these abnormalities and prevent retinal cell death.
Similar phenomena were also observed in streptozotocin-induced diabetic mouse models, which provided new insights into the pathogenesis of diabetic retinopathy and suggested that targeting the mtGSH transport system might become a new strategy for intervening in microvascular complications of diabetes.
Similar mechanisms may also exist in other microvascular complications such as diabetic nephropathy and diabetic peripheral neuropathy.
Glutathione metabolism regulation has demonstrated unique value in treatment of respiratory diseases, especially lung cancer
A team led by Chen Yiting from the School of Pharmaceutical Sciences at Fudan University has designed a nanoreactor based on polyunsaturated fatty acids (PUFA) DHA-N@M to target lung tumors through inhalation administration.
This innovative delivery system is composed of a macrophage cell membrane wrapped with DHA-SNO (a PUFA derivative that releases NO in response to GSH), and it can intelligently respond to the characteristics of high GSH levels in the tumor microenvironment.
When the nanoreactor enters the tumor cells, it can consume GSH, release NO and generate peroxynitrite (ONOO⁻) to synergiously induce ferroptosis. When used in combination with radiotherapy, it can achieve a tumor suppression rate of 93.91%.
This research not only provides a novel inhalable nanomedicine delivery system for lung cancer treatment, but also demonstrates the great potential of achieving precise treatment by taking advantage of the GSH responsiveness of tumors.
Geriatrics and anti-aging are another important direction for application of glutathione
A team led by Zhang Yujie, Luo Li and Xie Liangdi from the First Affiliated Hospital of Fujian Medical University discovered through multi-omics analysis that glutathione peroxidase 1 (GPX1) is a common potential intervention target for frailty and hypertension.
As a key antioxidant enzyme dependent on GSH, the activity changes of GPX1, through influencing REDOX balance, fatty acid and amino acid metabolism and other pathways, serve as a molecular link connecting two common diseases in the elderly.
This research not only provides A new perspective for understanding comorbidiasis in the elderly, but also screened out natural compounds such as chickatin A and epigallocatechin gallate through molecular docking as potential GPX1 regulators, laying the foundation for the development of anti-aging intervention measures.